Highly efficient synthesis of unsymmetrical 1,3-diynes from organoalane reagents and alkynyl bromides mediated by a nickel catalyst†
Abstract
Highly efficient and simple cross-coupling reactions of alkynylbromides with organoalane reagents for the synthesis of unsymmetrical 1,3-diynes derivatives using Ni(OAc)2 (2–5 mol%)/(o-furyl)3P (4–10 mol%) as a catalyst are reported. Excellent yields (up to 94%) were obtained for a wide range of substrates at rt or 60 °C for 2–3 h in Et2O or toluene.
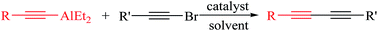


 Please wait while we load your content...
Please wait while we load your content...