Properties of a flavonol-based photoCORM in aqueous buffered solutions: influence of metal ions, surfactants and proteins on visible light-induced CO release†
Abstract
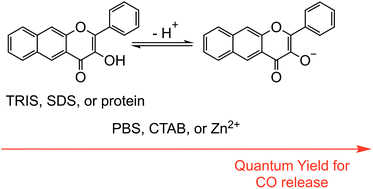
The properties of the extended flavonol 3-hydroxy-2-phenyl-benzo[g]chromen-4-one (2a) in DMSO : aqueous buffer solutions at pH = 7.4, including in the presence of metal ions, surfactants and serum albumin proteins, have been examined. Absorption and emission spectral studies of 2a in 1 : 1 DMSO : PBS buffer (pH = 7.4) indicate that a mixture of neutral and anionic forms of the flavonol are present. Notably, in 1 : 1 DMSO : TRIS buffer (pH = 7.4) only the neutral form of the flavonol is present. These results indicate that the nature of the buffer influences the acid/base equilibrium properties of 2a. Introduction of a Zn(II) complex of 2a− to a 1 : 1 DMSO : aqueous buffer (TRIS or PBS, pH = 7.4) solution produces absorption and emission spectral features consistent with the presence of a mixture of neutral 2a along with Zn(II)-coordinated or free 2a−. The nature of the anionic species present depends on the buffer composition. PBS buffered solutions (pH = 7.4) containing the surfactants CTAB or SDS enable 2a to be solubilized at a much lower percentage of DMSO (3.3–4.0%). Solutions containing the cationic surfactant CTAB include a mixture of 2a and 2a− whereas only the neutral flavonol is present in SDS-containing buffered solution. Compound 2a is also solubilized in TRIS buffer solutions at low cocentrations of DMSO (3.3%, pH = 7.4) in the presence of serum albumin proteins. Stern–Volmer analysis of the quenching of the inherent protein fluorescence indicates static binding of 2a to the proteins. The binding constant for this interaction is lower than that found for naturally-occurring flavonols (quercetin or morin) or 3-hydroxyflavone. Compound 2a binds to Site I of bovine and human serum albumin proteins as indicated by competition studies with warfarin and ibuprofen, as well as by docking investigations. The quantum yield for CO release from 2a (λirr = 419 nm) under aqueous conditions ranges from 0.0006(3) when the compound is bound to bovine serum albumin to 0.017(1) when present as a zinc complex in a 1 : 1 DMSO : H2O solution. Overall, the results of these studies demonstrate that 2a is a predictable visible light-induced CO release compound under a variety of aqueous conditions, including in the presence of proteins.


 Please wait while we load your content...
Please wait while we load your content...