Reductive dechlorination of endosulfan isomers and its metabolites by zero-valent metals: reaction mechanism and degradation products†
Abstract
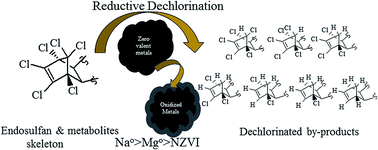
The widely used organochlorine pesticide endosulfan (ES) is extremely toxic to fishes, other aquatic species and mammals. Current evidence suggests that all known natural attenuation residues of ES, i.e., ES-metabolites, retain the original chlorinated skeleton of ES and are potential carcinogens. The objective of the present study was to identify the dechlorinated products formed during the reduction of ES-isomers, i.e., endosulfan-1 and endosulfan-2 and ES-metabolites, namely endosulfan sulfate (ES-S), endosulfan lactone (ES-L), endosulfan ether (ES-E) and endosulfan alcohol (ES-A), by various metallic surfaces. During dechlorination by nano zero-valent iron (NZVI), the mass spectra of the degradation products were consistent with the loss of one, two and three chlorine atoms from the parent molecules. During the interaction of ES-E with Mg0, the degradation products had mass spectra consistent with the loss of 1–6 chlorine atoms from the parent molecule. In all cases, dechlorination appears to occur through sequential electron transfer at the metallic surface. Tentative chemical structures for various degradation products were proposed. Synthesis of some of the observed degradation products was possible using ES-E as the starting molecule. 1H NMR, crystallographic (X-ray diffraction) and GC-MS analysis of the synthesized products provide final confirmation of the proposed chemical structures. A pathway for stepwise reductive dechlorination of ES-isomers and metabolites is proposed. Some of the degradation products identified in the present study being less chlorinated, may be less toxic and more amenable to subsequent biodegradation and ultimate mineralization in the natural environment.


 Please wait while we load your content...
Please wait while we load your content...