The synthesis of two long-chain N-hydroxy amino coumarin compounds and their applications in the analysis of aldehydes†
Abstract
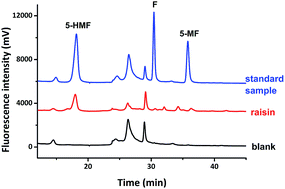
Herein, two N-substituted coumaryl hydroxylamines (compounds 1a and 1b) with long aliphatic chains were prepared in 8 steps with a novel synthetic protocol. They served as derivatization agents for aldehydes by the formation of nitrones under mild conditions, which can be readily analysed by LC-MS with good chromatographic performance, excellent fluorescent response, and high ionization strength. We successfully used compounds 1a and 1b in the analysis of furfuraldehydes in raisins, based on the derivatization reactions of standard furfural (F), 5-methylfurfural (5-MF) and 5-(hydroxymethyl)furfural (5-HMF) samples. It proved that they are excellent agents for the analysis of aldehydes in foodstuffs. The derivatization reaction between compound 1a and hexanal suggested that the applications of our designed derivatization agents can be further extended to the analysis of aliphatic aldehydes. This study demonstrated that the designed N-substituted coumaryl hydroxylamines are excellent probes for the analysis of various aldehydes which are important in multiple research areas.


 Please wait while we load your content...
Please wait while we load your content...