Kx(C2H8N2)yFe2−zS2: synthesis, phase structure and correlation between K+ intercalation and Fe depletion†
Abstract
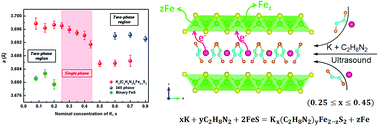
We report a new layered FeS compound Kx(C2H8N2)yFe2−zS2 synthesized by intercalating K and C2H8N2 into tetragonal FeS via a simple sonochemical route. This new compound crystallizes in a body-centered tetragonal unit cell, with the [K(C2H8N2)] and [FeS] layers alternately stacking along the c direction. The nominal concentration of K, x, can be adjusted from 0.25 to 0.45, and the lattices a and c contract from 3.6971(9) and 20.667(5) Å to 3.691(1) and 20.566(7) Å, respectively. When x < 0.25, the parent FeS is residual and when x > 0.45, K reacts with FeS directly to form K2Fe4S5 impurity. It is found that the C2H8N2 molecule has been co-intercalated in between the [FeS] layers along with K, evidenced by its content, y, having a linear dependence with x. Measurements indicate that Kx(C2H8N2)yFe2−zS2 is a semiconductor and it shows a weak ferrimagnetism below 50 K. More importantly, Fe depletion resulting from the charged K+ intercalation was revealed by composition analysis, which leads to the formation of disordered Fe vacancies in the [FeS] layers and hence hinders the enhancement of original superconductivity in the FeS parent.


 Please wait while we load your content...
Please wait while we load your content...