Decolorization of Color Index Acid Orange 20 buffer solution using horseradish peroxidase immobilized on modified PAN-beads
Abstract
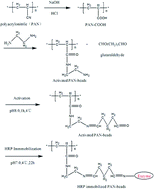
In the present work, horseradish peroxidase (HRP) is utilized to be immobilized onto polyacrylonitrile based beads (PAN-beads) for decolorization of Color Index (C. I.) Acid Orange 20 (AO20) in aqueous solution. Sodium hydroxide and hydrochloric acid were used to treat PAN-beads to obtain carboxyl groups, followed by being modified with ethanediamine to get more amino groups, and then were activated with glutaraldehyde. The enzyme was immobilized onto the activated beads by covalent crosslinking. Optimum factors for decolorization of AO20 were investigated both on free horseradish peroxidase (F-HRP) and immobilized horseradish peroxidase (I-HRP), including pH values, H2O2 amount, enzyme amount, temperature and so on. Under optimum conditions, percent decolorization was about 90% or 98% respectively for F-HRP or I-HRP. A kinetic study showed that F-HRP possessed more affinity compared to I-HRP, but reusability experiments clarified that I-HRP was cost-efficient as about 90% decolorization was obtained after three cycles. The human normal hepatocytes cell line (L02 cell line) was utilized to assess cytotoxicity of metabolites, and the result was acceptable.


 Please wait while we load your content...
Please wait while we load your content...