Synthesis, biological evaluation and molecular docking of spirofurochromanone derivatives as anti-inflammatory and antioxidant agents†
Abstract
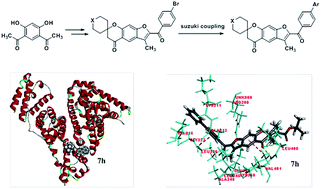
A series of 2′-substituted-3′-methylspiro[cyclohexane-1,7′-furo[3,2-g]chroman]-5′(7′H)-one, 5a–i and 7a–u have been synthesized using an eco-friendly approach to attain good yields in a shorter reaction time. The structures of novel compounds were characterized by IR, 1H NMR, 13C NMR and mass spectrometry analysis. All the synthesized compounds were evaluated for their biological activity. Compounds 5a, 5b, 5c, 5d, 5e, 7g, 7h, 7j, 7l, 7n and 7q were found to have better anti-inflammatory activity in the albumin denaturation technique. All compounds exhibited good antioxidant activity by DPPH radical scavenging assay and most compounds showed activity in a hydrogen peroxide assay. Molecular docking scores as well biological assays results suggested that compound 7h has better anti-inflammatory activity among the synthesized compounds.


 Please wait while we load your content...
Please wait while we load your content...