Ferrous complexes supported by sterically encumbered asymmetric bis(arylimino)acenaphthene (BIAN) ligands: synthesis, characterization and screening for catalytic hydrosilylation of carbonyl compounds†
Abstract
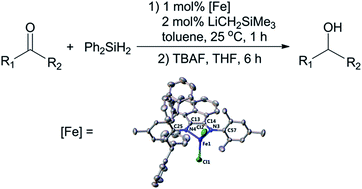
Six ferrous chloride complexes ((Ar-BIANX)FeCl2: Ar = 2,6-diisopropylphenyl (Dipp), X = F (1), Cl (2), Me (3); Ar = mesityl (Mes), X = F (4), Cl (5), Me (6)) supported by sterically bulky asymmetric bis(arylimino)acenaphthene (BIAN) ligands were prepared through the treatment of anhydrous FeCl2 with the corresponding ligands in a molar ratio of 1 : 1. The compounds were characterized by X-ray crystallography, IR, NMR and electrochemical methods. This series of complexes represents rare examples of structurally characterized iron compounds bearing asymmetric bidentate BIAN ligands. The complexes were tested for catalytic hydrosilylation of aldehydes and ketones at room temperature, and moderate to good yields of alcohol derivatives were obtained after hydrolysis workup.


 Please wait while we load your content...
Please wait while we load your content...