Sensing of hydrogen peroxide and glucose in human serum via quenching fluorescence of biomolecule-stabilized Au nanoclusters assisted by the Fenton reaction†
Abstract
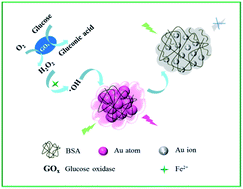
Fe2+ can act as a catalyst to disproportionate hydrogen peroxide (H2O2) to produce extremely reactive hydroxyl radicals (˙OH) through the so-called Fenton reaction. Combining this reaction with the prominent sensitive nature of gold nanoclusters (Au NCs), we present herein a simple strategy of sensitive and rapid detection of H2O2. Compared with H2O2, the produced hydroxyl radical exhibits a much stronger oxidizing ability, and therefore could lead to a more efficient oxidation of the Au NCs and an improved sensitivity and oxidation rate. The results indicate that the detection limit for the determination of H2O2 was 0.2 μM (signal/noise = 3) and the linear range was 0.4–12 μM. Furthermore, in combination with the specific catalytic effect of glucose oxidase, the present sensing strategy can be successfully expanded to detect glucose in blood. The preliminary results are in good agreement with those provided by the hospital, which suggests the generalizability and great potential of the Au NCs/Fenton hybrid system for research and clinical diagnosis of diabetes.


 Please wait while we load your content...
Please wait while we load your content...