Thermodynamic stabilization of semiclathrate hydrates by hydrophilic group
Abstract
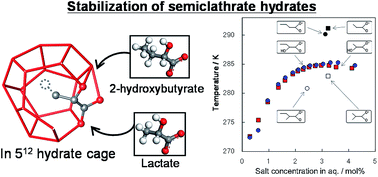
Introducing hydrophilic groups into carboxylates is a way to modify semiclathrate hydrate frameworks and change the properties of the hydrates. In this study, we report the characterization of semiclathrate hydrates formed by tetra-n-butylammonium (TBA) 2-hydroxybutyrate (2HB). In addition, TBA lactate and the TBA 2HB salt formed stable hydrate crystals, which basically had rectangular columnar shapes. We performed equilibrium measurements and calorimetry. The melting temperature and fusion heat of the TBA 2HB hydrate crystals were 285.3 K and 177 kJ kg−1, respectively. A comparison with other carboxylate anions showed that the substitution of hydrogen atom at the 2-position in the carbon chain by a hydrophilic hydroxy group stabilizes the hydrates more than that by hydrophobic methyl group, which is the case for alcohols in clathrate hydrates. The phase equilibrium data for a number of semiclathrate hydrates were compared. A rough trend of temperature depending on type of guest anions was observed, but it is unclear if there are other correlating factors.

 Please wait while we load your content...
Please wait while we load your content...