Enantioselective Barbier-type allylation of ketones using allyl halide and indium in water†
Abstract
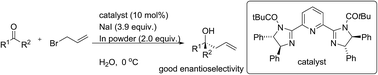
We disclose herein an efficient enantioselective Barbier-type allylation of ketones using allyl halide and indium metal in water. The reaction was catalysed by chiral bis(imidazoline) to afford homoallylic alcohols having quaternary stereocenters in good yield with moderate to good enantioselectivity. Based on experimental investigation, a possible transition state has been proposed to explain the origin of the asymmetric induction.


 Please wait while we load your content...
Please wait while we load your content...