Challenging cyclopropanation reactions on non-activated double bonds of fatty esters†
Abstract
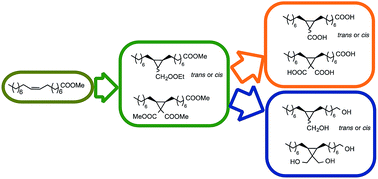
Monounsaturated fatty esters with non-activated double bonds have been made to react with two different diazocompounds, ethyl diazoacetate (EDA) and dimethyl diazomalonate (DDM), in copper and rhodium catalyzed cyclopropanation reactions. The corresponding cyclopropanes have been obtained with quantitative or nearly quantitative yields (>99%) in all the cases, with only the exception of the reaction of methyl elaidate, an E alkene, with the less reactive DDM. For the first time, the two stereoisomers of ethyl (2R*,3S*)-2-(7-methoxycarbonyl)heptyl-3-octylcyclopropane-1-carboxylate, obtained from the reaction of methyl oleate and EDA, have been separated and fully characterized. Finally, the diesters and triesters with cyclopropane structure have been reduced to diols and triols, and hydrolyzed to the corresponding diacids and triacids, to demonstrate the potential usefulness of this methodology to prepare monomers from renewable sources.


 Please wait while we load your content...
Please wait while we load your content...