Semisynthesis of some matrine ether derivatives as insecticidal agents†
Abstract
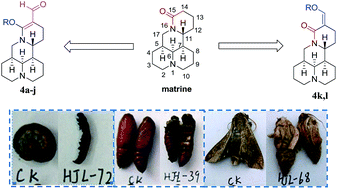
In continuation of our program to discover new potential natural-product-based crop protection agents, we synthesized a series of 14-formyl-15-aryloxy/methoxymatrine and 14-aryloxymethylidenylmatrine derivatives as pesticidal agents by structural modification of matrine, a biorenewable quinolizidine alkaloid isolated from Sophora flavescens. The structural assignment was based on spectroscopic and X-ray analysis data. Their pesticidal activities were carried out against two typically crop-threatening agricultural insect pests, Mythimna separata Walker and Plutella xylostella Linnaeus. Compounds 4i and 4k exhibited more potent oral toxicity than matrine against 3rd-instar larvae of P. xylostella. As compared with matrine, all derivatives displayed a growth inhibitory property against early 3rd-instar larvae of M. separata, and in particular compounds 4i–k displayed more promising insecticidal activity than toosendanin. Some interesting results of structure–activity relationships were also observed.


 Please wait while we load your content...
Please wait while we load your content...