Understanding the chemical state of palladium during the direct NO decomposition – influence of pretreatment environment and reaction temperature†
Abstract
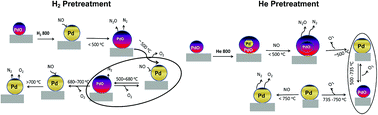
Direct decomposition of NO into N2 and O2 offers an ideal solution to the abatement of NO from automotive and various combustion processes. Although its decomposition is thermodynamically favorable (ΔG = −86.6 kJ mol−1 at 25 °C), yet kinetically hindered due to a high activation barrier of 335 kJ mol−1, making use of catalyst indispensable. Supported Pd catalysts have been investigated recently for the reaction ranging from 300 °C to 1000 °C. However, the function of palladium during the reaction at various reaction temperatures is still unclear. SiO2 supported palladium was studied as a model catalyst system for direct NO decomposition over temperatures ranging from 100 to 800 °C both in dynamic and steady state mode to understand the changes in the palladium chemical state with respect to the reaction temperature. Three different types of catalyst compositions of palladium namely Pd, PdO and mixture of Pd–PdO, were created on silica surface by controlling pretreatment environments for systematic comparison. Combining characterization and catalytic performance, it has been revealed that the reaction in the lower temperature region (<500 °C), is not catalytic and NO chemically reacts with metallic Pd and forms PdO. The oxidation ability of metallic Pd is sensitive to the particle size of the metallic Pd. The catalyst pretreated in helium yields Pd–PdO composite structure and higher active metallic surface area compared to the hydrogen pretreated catalyst and exhibited higher NO conversion at lower reaction temperatures (<500 °C). At temperatures higher than 500 °C the PdO converts to metallic Pd by releasing oxygen, resulting in stable direct NO activity. Our results suggest retarding metallic Pd oxidation is key factor to develop a sustainable catalyst for direct NO decomposition at lower temperatures.


 Please wait while we load your content...
Please wait while we load your content...