Assessing the effective factors affecting the conformational preferences and the early and late transition states of the unimolecular retro-ene decomposition reactions of ethyl cyanate, ethyl thiocyanate and ethyl selenocyanate†
Abstract
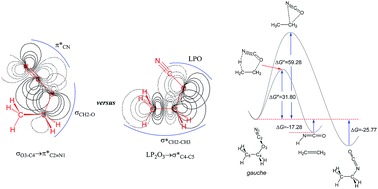
The structural and conformational properties of ethyl cyanate (1), ethyl thiocyanate (2), and ethyl selenocyanate (3) and also their corresponding unimolecular retro-ene decomposition and isomerization reactions were investigated by means of G3(MP2) and MP2/6-311++G** methods and natural bond orbital interpretations. We assessed the role and contributions of the hyperconjugative interactions on the conformational preferences of compounds 1–3 by the deletion of the orbitals overlapping from the Fock matrices of the gauche- and anti-conformations, where the results obtained showed that the deletion of these hyperconjugative interactions from the Fock matrices leads to an increase in the anti-conformations preferences, revealing the significant impacts of the hyperconjugative interactions on the gauche-conformations preferences going from compound 1 to compound 3. The hyperconjugative interactions, Pauli exchange-type repulsions (PETR), and electrostatic model associated with the dipole–dipole interactions were in favor of the gauche-conformation of compound 1. Contrary to the conclusions published in the literature, there is no fact that justifies the anti-conformation preference in compound 1. Accordingly, we concluded that there are two rotamers (i.e., gauche- and anti-conformations) with literally the same populations for compound 1. The unimolecular retro-ene decomposition reactions of compounds 1–3 were more feasible than their corresponding cyanate → isocyanate isomerization reactions. The Pauli exchange-type repulsions have determining impacts on the retro-ene decomposition reactions of these compounds. The correlations between the activation Gibbs free energies and the advancements of the transition states (δBav) of the retro-ene decomposition reactions of compounds 1–3 according to the Hammond–Leffler postulate were also analyzed. Interestingly, the variations of the bond lengths in the transition state structures of the retro-ene decomposition reactions of compounds 1–3 were in accordance with the Hammond–Leffler postulate.


 Please wait while we load your content...
Please wait while we load your content...