Experimental and theoretical studies on solvation in aqueous solutions of ionic liquids carrying different side chains: the n-butyl-group versus the methoxyethyl group†
Abstract
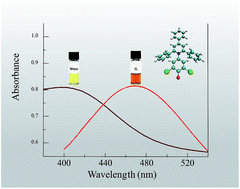
We used solvatochromic compounds to probe solvation in mixtures of water, W, and four ionic liquids (ILs), 1-R-3-methylimidazoliumX, where R = n-butyl or methoxyethyl and X = acetate and chloride; these are denoted as (C4MeImAc), (C3OMeImAc), (C4MeImCl), and (C3OMeImCl). Our aim was to investigate the effects on solvation when an ether linkage is substituted for a –CH2– group in the IL side chain. We used the solvatochromic probes 2,6-dichloro-4-(2,4,6-triphenylpyridinium-1-yl)phenolate (WB) and 5-nitroindoline, 1-methyl-5-nitroindoline to determine the solvent polarity, ET(WB), and Lewis basicity, SB, respectively. From UV-Vis spectral data, we calculated ET(WB) as a function of the water mole fraction (χW) at different temperatures; from 15 to 60 °C for WB in IL–acetate–W; 25 °C for SB and WB in IL–chloride–W. For all IL–W mixtures, the dependence of ET(WB) on χW is non-linear and, surprisingly, shows negligible dependence on the nature of the side chain. Values of the SB of IL–W were higher for C4MeImX–W than for C3OMeImX. A rationale for these results is the deactivation of the ether oxygen due to the formation of intramolecular hydrogen bonds with the hydrogens of the imidazolium ring. Our hypothesis is confirmed by quantum chemistry and molecular dynamics calculations (energy of the conformers and radial distribution functions), density, and 1H NMR data (chemical shifts, line widths). We attributed the non-linear dependence of the solvatochromic parameters on χW to preferential solvation of the dyes. We treated ET(WB) data with a model that includes the formation of the complex solvent IL–W. Equilibrium constants for solvent exchange in the solvation layer of WB were calculated; their values showed that IL–W is the most efficient solvent species present.


 Please wait while we load your content...
Please wait while we load your content...