Synthesis and evaluation of novel F-18-labeled pyrimidine derivatives: potential FAK inhibitors and PET imaging agents for cancer detection†
Abstract
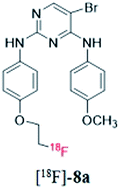
Based on computer-assisted drug design, a series of novel pyrimidine derivatives was successfully synthesized and characterized by 1H NMR, 13C HNMR, and MS spectra. All the new compounds were evaluated for their activity against focal adhesion kinase and showed low IC50 values in comparison with control drugs. In particular, for compound 8i, its IC50 value was 0.060 μM, suggesting its advantage as a focal adhesion kinase inhibitor. To evaluate the potentiality of these compounds as PET imaging agents in cancer detection, compounds 8a, 8c, 8h, and 8i were successively labeled with 18F. The four 18F-labeled pyrimidine derivatives showed appropriate log P values and high stability in physiological saline and mouse plasma. Noticeably, compound [18F]-8a with a 4-methoxyl group at the benzene ring exhibited good in vivo biodistribution data in mice bearing the S180 tumor, which promoted a further microPET imaging study of compound [18F]-8a. The microPET image of [18F-8a] administered into the S180 tumor-bearing mice acquired at 60 min post-injection illustrated that the uptake in S180 tumor was obvious. These results suggested that compound [18F]-8a might be a new probe for PET tumor imaging.


 Please wait while we load your content...
Please wait while we load your content...