Synthesis and antioxidant activity of 1,3,4-oxadiazoles and their diacylhydrazine precursors derived from phenolic acids†
Abstract
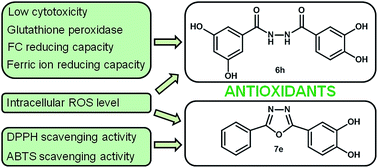
Eight 1,3,4-oxadiazole derivatives containing phenolic acid moieties (7a–h) and eight of their diacylhydrazine precursors (6a–h) were synthesized, characterized using spectroscopic methods and examined by scavenging of stable DPPH (2,2-diphenyl-1-picrylhydrazyl) radicals. The most potent phenolic 1,3,4-oxadiazoles showed better DPPH scavenging activity in comparison with their corresponding diacylhydrazine precursors as a result of participation of both aromatic rings and a 1,3,4-oxadiazole moiety in resonance stabilization of the formed phenoxyl radical. Four diacylhydrazines (6d, 6e, 6g, and 6h) and four 1,3,4-oxadiazoles (7d, 7e, 7g and 7h) with the best DPPH scavenging activity, were chosen for further evaluation of their antioxidant potential through various assays. The investigated compounds exerted pronounced ABTS radical scavenging capacity, moderate to good H2O2 scavenging properties and strong ferric ion reducing capacity. Further in vitro evaluation of the antioxidant properties of the most active compounds demonstrated their protective effects in normal lung fibroblasts MRC-5 against hydrogen peroxide induced oxidative stress. Diacylhydrazine 6h increased two times the activity of glutathione peroxidase in treated cells in comparison with a control sample and did not affect the superoxide dismutase activity.


 Please wait while we load your content...
Please wait while we load your content...