The evolution of comprehensive strategies for furanoid glycal synthesis and their applications†
Abstract
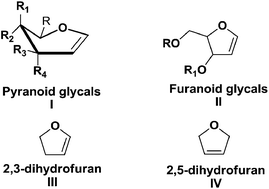
Cyclic enol ether frameworks, especially stereochemically pure furanoid and pyranoid glycals are well known highly functionalized chiral building blocks. Furanoid glycals have been shown to possess great potential, as they have been used as key intermediates for the synthesis of structurally diverse molecules including natural products with various biological activities since their discovery in 1963 by Ness and Fletcher. In this review on furanoid glycals, efforts are made to exhaustively compile the work centered on synthesis and utility of furanoid glycals since the inception to date. This review aims to highlight the importance of furanoid glycals and the strategies developed over the years for their synthesis. Attention has also been focused on the use of these furanoid glycals toward the synthesis of natural and unnatural products including C-and N-nucleosides of biological importance. Apart from that, efforts are also devoted to cover the significant applications of these furanoid glycals for stereoselective synthesis of various cyclic and acyclic key intermediates of significant interest.
- This article is part of the themed collection: 2017 Review articles


 Please wait while we load your content...
Please wait while we load your content...