Trifluoromethanesulfonic acid-based DESs as extractants and catalysts for removal of DBT from model oil
Abstract
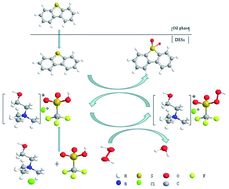
A series of deep eutectic solvents (DESs) of ChCl/XCF3SO3H (X from 1.0 to 2.0) were synthesized by stirring a mixture of choline chloride (ChCl) and trifluoromethanesulfonic acid (CF3SO3H) at room temperature. The DESs were characterized by Fourier transform infrared (FT-IR) and 1H nuclear magnetic resonance (1H NMR). The oxidative desulfurization of model oil was investigated using ChCl/1.5CF3SO3H as a catalyst and extraction agent, and H2O2 as the oxidant. Some reaction parameters such as type of DES, molar ratio of CF3SO3H and ChCl in DESs, H2O2 dose, reaction temperature, DES dose and type of sulfur compound were investigated. Under the optimum conditions, the removal rate of dibenzothiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) can reach up to 98.65% and 96.8%, respectively. After five recycling runs, the removal rate of DBT can still reach 97.16%.


 Please wait while we load your content...
Please wait while we load your content...