The degradation and persistence of five pharmaceuticals in an artificial climate incubator during a one year period†
Abstract
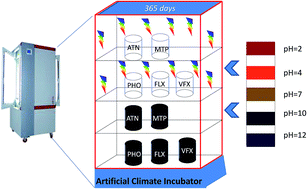
The degradation and persistence of the five pharmaceuticals atenolol, metoprolol, propranolol, fluoxetine and venlafaxine at an initial concentration of 10 mg L−1 was studied at different pH values under fluorescent light and darkness in an artificial climate incubator during a one-year period. The results suggested that the degradation process of the target pharmaceuticals followed pseudo first-order degradation kinetics. Propranolol was degraded at a higher rate, with a half-life ranging from 5.7 to 28.5 d. The degradation rates of propranolol and venlafaxine increased with increasing pH value, while the other pharmaceuticals did not show a clear pH correlation, with the highest degradation rate at pH 7 for atenolol, pH 2 for metoprolol and pH 12 for fluoxetine. Both photodegradation and hydrolysis should contribute to the degradation. Typical transformation products were detected and were identified as metoprolol acid, α-hydroxymetoprolol, norfluoxetine and O-desmethylvenlafaxine. The findings could not only help to understand the degradation and fate of pharmaceuticals but also provide the fundamental data for persistence assessments.


 Please wait while we load your content...
Please wait while we load your content...