Leaching of fluorine and rare earths from bastnaesite calcined with aluminum hydroxide and the recovery of fluorine as cryolite
Abstract
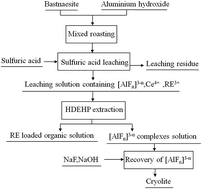
In order to avoid fluorine loss and contamination, and recover the fluorine resource in bastnaesite, a new process of decomposing bastnaesite with aluminum hydroxide followed by sulfuric acid leaching was proposed. The calcination mechanism was discussed by TG-DSC and XRD analyses. Fluorine could be taken up by Al2O3, and the discharge of fluorine was prevented. Leaching from calcined ore was achieved with dilute sulfuric acid, and the parameters affecting the leaching rates of fluorine and rare earths (REs) were investigated. It shows that the leaching rates of F, Ce and total RE of 92.71%, 98.92% and 98.61% could be obtained under the conditions of n(Al)/n(F) 1/2, calcination temperature 500 °C, calcination time 1 h, sulfuric acid concentration 3 mol L−1, leaching temperature 90 °C and leaching time 1 h. Fluorine could be separated from REs after extraction, and was recovered as Na3AlF6 with the recovery rates of fluoride and aluminum greater than 96%. XRD and SEM analyses indicated that the monoclinic phase cryolite was obtained.


 Please wait while we load your content...
Please wait while we load your content...