Laccase-catalyzed green synthesis and cytotoxic activity of novel pyrimidobenzothiazoles and catechol thioethers†‡
Abstract
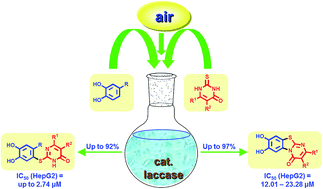
The laccase-catalyzed reaction between unsubstituted catechol and 2-thioxopyrimidin-4(1H)-ones using aerial O2 as the oxidant delivers novel pyrimidobenzothiazoles with high yields in an aqueous solvent system under mild reaction conditions. With 4-substituted catechols, catechol thioethers are formed exclusively. The synthetic protocols developed provide a sustainable approach for these compound classes. In addition, the cytotoxicity of the products against HepG2 cell line is reported. Most compounds exhibit antiproliferative activities with IC50 values at the micromolar level. A structure–activity relationship study will facilitate the further development of these compounds as cytotoxic agents.
- This article is part of the themed collection: Sustainable synthesis


 Please wait while we load your content...
Please wait while we load your content...