Design, synthesis, antibacterial and insecticidal activities of novel N-phenylpyrazole fraxinellone hybrid compounds†
Abstract
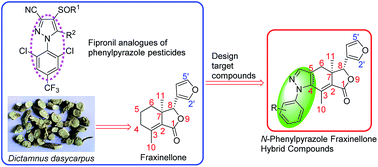
In continuation of our research aimed at the discovery and development of natural product-based antimicrobial and insecticidal agents, a series of 20 novel N-phenylpyrazole fraxinellone hybrid compounds were prepared and evaluated for their antibacterial and insecticidal activities. Two key steric configurations of compounds 4h and 4r were unambiguously determined by X-ray crystallography. Although only compound 4g showed better antibacterial activity against Bacillus subtilis with MIC value of 4 μg mL−1, than insecticidal activity, compounds 4n, 4o and 4t exhibited more promising insecticidal activity with final mortality rates (FMRs) of >60%, when compared with their precursor fraxinellone and the positive control toosendanin. This suggested that introducing polyhalogenated phenylpyrazole at C-4 and C-5 positions of fraxinellone could lead to more promising insecticidal derivatives than monohalogenated phenylpyrazole, especially introducing N-(2-chloro-4-fluoro-phenyl)pyrazole.


 Please wait while we load your content...
Please wait while we load your content...