Stability and clusterization of hydrogen-vacancy complexes in B2-FeAl: insight from hydrogen embrittlement
Abstract
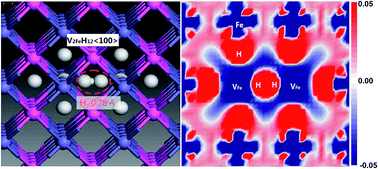
Little is known about hydrogen-vacancy interactions and their contributions to hydrogen embrittlement (HE) in iron aluminides. H-induced vacancy formation, stability and clusterization of hydrogen-vacancy complexes (VFeHn) in B2-FeAl were studied via density functional theory (DFT) and thermodynamic formalism. The presence of an interstitial H atom in FeAl forms superabundant Fe-vacancies. The H atoms are more likely to be trapped around the Fe-vacancies than diffuse from one octahedral interstitial site to another. One Fe-vacancy can trap at most six H atoms to form VFeHn complexes with H atoms occupying the six first-nearest-neighbor (1NN) Oct2Fe–4Al sites of VFe one by one; the H–H distances are 1.920–2.785 Å. The VFeH6 complex is the major complex under ambient conditions and prefers to grow larger by clusterization of V2FeH12 units along 〈100〉 and {100} with internal H2 molecules closely associated with the crack along the {100} planes. Thus we propose a mechanism of isotropic hydrogenated vacancy-cluster induced HE: hydrogen addition-induced isotropic V2FeH12〈100〉 clusters of line and planar shapes are embryos for the formation of cracks and H2 bubbles. This grows ever bigger as a function of H concentration and eventually leads to the macroscopic failure observed experimentally.


 Please wait while we load your content...
Please wait while we load your content...