Cross C–S coupling reaction catalyzed by copper(i) N-heterocyclic carbene complexes†
Abstract
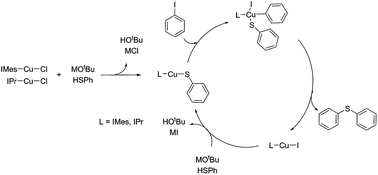
Copper(I) N-heterocyclic carbene has a good activity towards aryl halides and was used as a model complex to study the catalytic cycle of Cu(I) to catalyze the cross C–S coupling reaction because the N-heterocyclic carbene has a strong electron donating property, and ligand dissociation can be avoided. Free radical scavenger cumene does not retard the yield of the reaction indicating that the catalytic reaction goes through a non free radical path. Switching the solvent from toluene to DMF lowered the yield of the reaction. DFT calculation shows that the activation of aryl halide is the rate determining step, and the activation energy is higher for the reaction in DMF than in toluene. A plausible catalytic cycle is proposed with the support of DFT calculation.


 Please wait while we load your content...
Please wait while we load your content...