Fabrication of two-layer dissolving polyvinylpyrrolidone microneedles with different molecular weights for in vivo insulin transdermal delivery
Abstract
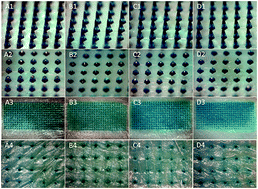
Transdermal drug delivery is a convenient route to transport pharmaceuticals. However, its application is limited to a few compounds. Microneedle (MN) patches have demonstrated a high efficiency for delivering poorly permeable drugs and gained significant attention due to the advantages of being painless and convenient for patients as well as providing efficient delivery. This study presents dissolvable polyvinylpyrrolidone (PVP)-based MNs that do not require needle removal while providing the rapid release of encapsulated insulin. PVP with two molecular weights, PVP10 and PVP360, were used to fabricate the needle portion of the MNs, and PVP360/sodium carboxymethyl cellulose (CMC) was used for preparation of the backing layer. The detailed characteristics and in vitro skin penetration capability of a series of PVP360/PVP10 MNs were analyzed. The penetration depths of PVP MNs were evaluated using optical coherence tomography. The PVP360/PVP10 = 1 : 3 MN patch was chosen for ex vivo and in vivo studies. Ex vivo drug release profiles show that the permeation efficiency of Lissamine green B (LGB)- and fluorescein isothiocyanate-labeled BSA (FITC-BSA)-loaded PVP MN patches was much higher than the groups of patches without MNs. Furthermore, to estimate the feasibility of PVP MNs for diabetes treatment, insulin-loaded PVP MN patches were administered to diabetic mice to evaluate glycemic control. The relative pharmacologic availability revealed that the MN patch has an immediate and effective effect on hypoglycemic administration. This study demonstrates that dissolvable PVP MNs may serve as a promising device to deliver macromolecules or protein drugs for efficient transdermal drug delivery.


 Please wait while we load your content...
Please wait while we load your content...