Synergism between non-ionic and cationic surfactants in a concentration range of mixed monolayers at an air–water interface
Abstract
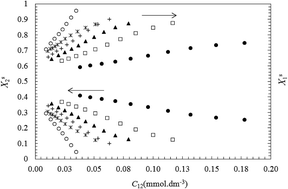
The surface properties and adsorption behavior of mixed binary surfactants containing alkylpyridinium chloride (CnPC, n = 14 and 16) and Triton X-100 (TX100) have been studied at 298.15 K. The values of the molecular interaction parameter (βs) and the mole fraction of components (Xsi) at the air–water interface in the pre-micellar region were calculated on the basis of Rosen's model. In the new approach, we proposed to estimate surface parameters (βs, Xsi, Γtot, Atot, ΓTX100 and ΓCnPC) in a pre-micellar region, using different fixed values of the surface tension instead of a single-value of surface tension. Then, the influence of surface tension on the surface properties was investigated. The interaction parameter (βs) is negative at all compositions showing a synergistic effect between the components. The βs was found to considerably decrease (less negative values) with decreasing surface tension due to more migration of TX100 to the surface layer and a reduction in electrostatic self-repulsion between head groups in the ionic surfactant. Also, the total surface area (Atot) at the air–water interface decreases with increasing Xs1 (mole fraction of TX100 in mixed monolayer) or decreasing surface tension due to the dilution effect on mixing.


 Please wait while we load your content...
Please wait while we load your content...