Convenient iodine-mediated aminoselenation of alkenes using benzotriazoles as nitrogen sources†
Abstract
A new and convenient procedure mediated by I2 was developed for the preparation of aminoselenides bearing the benzotriazole structure using alkenes, diselenides, and benzotriazoles. In this protocol, molecular I2 first reacts with diselenides to form active electrophilic selenium species, following an electrophilic addition to afford the corresponding aminoselenides. This aminoselenation of alkenes requires mild reaction conditions and is a simple procedure, and it provides the products with high regioselectivity and in good yields, which extends the synthetic application of molecular iodine in organic synthesis.
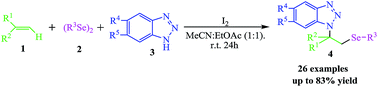


 Please wait while we load your content...
Please wait while we load your content...