Thermo-reversible sol–gel transition of aqueous solutions of patchy polymers†
Abstract
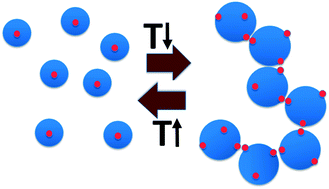
While aqueous solutions of several amphiphilic thermo-reversible polymers show gelation upon heating, there are fewer examples of polymer solutions that exhibit gelation when cooled. This paper reports an interesting phenomenon of abrupt thermoreversible gelation of aqueous solutions of a hydrophobically modified polymer upon cooling. A high molecular weight precursor copolymer (PCP, 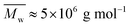 ) of N,N-dimethylacrylamide (70 mol%) and acrylic acid (30 mol%) was modified by reacting 10 mol% of the acrylic acid groups with n-dodecyl amine to form a hydrophobically modified copolymer (HMCP). The composition of the copolymer was ascertained using NMR spectroscopy. Cooling the solution of PCP at a controlled rate resulted in a gradual increase in its low shear viscosity as dictated by the flow activation energy. In contrast, cooling the solution of HMCP under identical conditions resulted in an abrupt and large non-Arrhenius increase in viscosity at a specific transition temperature, which decreased with decrease in polymer concentration. Fluorescence measurements and dynamic light scattering data showed that abrupt gelation happened upon cooling, when polymer coils percolate accompanied with concomitant transition in chain conformation from compact micellar coils formed by intra-chain hydrophobic associations to swollen polymer coils connected by inter-chain hydrophobic interactions.
) of N,N-dimethylacrylamide (70 mol%) and acrylic acid (30 mol%) was modified by reacting 10 mol% of the acrylic acid groups with n-dodecyl amine to form a hydrophobically modified copolymer (HMCP). The composition of the copolymer was ascertained using NMR spectroscopy. Cooling the solution of PCP at a controlled rate resulted in a gradual increase in its low shear viscosity as dictated by the flow activation energy. In contrast, cooling the solution of HMCP under identical conditions resulted in an abrupt and large non-Arrhenius increase in viscosity at a specific transition temperature, which decreased with decrease in polymer concentration. Fluorescence measurements and dynamic light scattering data showed that abrupt gelation happened upon cooling, when polymer coils percolate accompanied with concomitant transition in chain conformation from compact micellar coils formed by intra-chain hydrophobic associations to swollen polymer coils connected by inter-chain hydrophobic interactions.


 Please wait while we load your content...
Please wait while we load your content...