Alkaloids and polyketides from the South China Sea sponge Agelas aff. nemoechinata†
Abstract
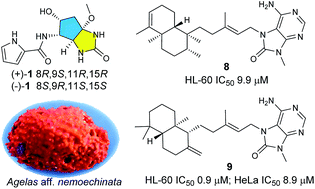
A chemical investigation of the South China Sea sponge Agelas aff. nemoechinata yielded three pairs of new enantiotopic pyrrole alkaloids nemoechines A–C (1–3), one new diterpene-adenine alkaloid nemoechine D (8), and one new polyketide nemoechioxide A (10), together with nine known analogues (4–7, 9, 11–14). Compounds 1–3 were initially obtained as racemates and were further separated by chiral HPLC chromatography to afford the three pairs of enantiomers. Their structures including absolute configurations of compounds 1–3 were elucidated on the basis of comprehensive spectroscopic analysis and quantum chemical calculation. Nemoechine A (1), possessing an unusual cyclopentane-fused imidazole ring system, represents the first monomeric precursor of nagelamide J. Compounds 8 and 9 showed cytotoxicity against HL-60 cell lines with IC50 values of 9.9 and 0.9 μM, respectively, and compound 9 was also cytotoxic against HeLa cell lines with IC50 value of 8.9 μM.


 Please wait while we load your content...
Please wait while we load your content...