Switching of the π-electronic conjugations in the reduction of a dithienylethene-fused p-benzoquinone†
Abstract
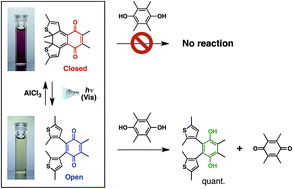
The electron accepting character of a dithienylethene-fused p-benzoquinone derivative is significantly reduced upon ring-closing isomerization. Visible light unlocks the π-electronic conjugation of the quinone so it can be utilized for a light-driven oxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...