The concise synthesis and biological evaluation of C-glycosyl chalcone analogues inspired by the natural product aspalathin†
Abstract
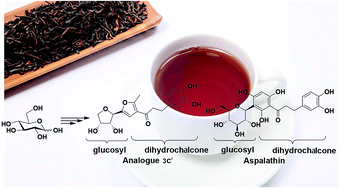
We describe the synthesis and biological evaluation of C-glycosyl chalcone analogues of aspalathin. The DPPH radical scavenging method was used to detect their antioxidant activity and an MTT colorimetric assay was employed to test the anticancer activity against Hep-G2 liver carcinoma cells and MCF-7 human breast carcinoma cells. The antioxidant ability between C-furanosides and C-pyranosides was compared. Compound 3c′ was shown to be the most promising compound with good antioxidant ability and the ability to inhibit Hep-G2 and MCF-7 cells, with IC50 values of 4.9 μM and 19 μM, respectively.


 Please wait while we load your content...
Please wait while we load your content...