Vascularization of LBL structured nanofibrous matrices with endothelial cells for tissue regeneration†
Abstract
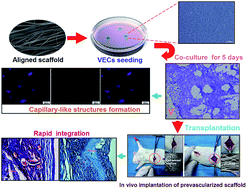
To engineer functional vascular structures for reconstruction in tissue engineering, we evaluated the feasibility of layer-by-layer (LBL) isotropic and anisotropic structured poly(ε-caprolactone) (PCL)/cellulose based nanofibers via electrospinning and LBL techniques in this study. The morphology of both fibers was analyzed using field emission scanning electron microscopy (FE-SEM) and atomic force microscopy (AFM). The aligned nanofibrous scaffold surface was nonthrombogenic as assessed using a platelet adhesion test, and the antithrombogenicity of modified nanofibrous mats was increased greatly with increased coating bilayers. Besides, human umbilical vein endothelial cells (HUVECs) were then seeded onto the LBL structured nanofiber meshes and analyzed for cell adhesion, proliferation and migration by FE-SEM, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cell tracking and cell migration assay. Moreover, the phenotypic expressions of HUVECs on LBL structured nanofibrous matrices with either isotropic or anisotropic fiber organizations were studied by immunofluorescent staining. Our data found that aligned nanofibers could guide morphogenesis and regulate cytoskeleton organization of HUVECs, and further promote in vitro prevascularization by facilitating phenotype-related protein expression and capillary-like tube formation as compared to randomly oriented nanofibers. Furthermore, the implantation in vivo of aligned composite scaffolds seeded with VECs demonstrated that the promoted host vessel infiltrated deep into the scaffolds and integrated with in vitro prefabricated vascular structures with increasing coating bilayers. Together, these findings supported our notion that the combination of aligned nanofibrous scaffolds and prevascularization could therefore serve as a promising strategy for the development of implantable functional vascular grafts by promoting rapid vascularization.


 Please wait while we load your content...
Please wait while we load your content...