Synthesis and characterization of boron-doped ordered mesoporous carbon by evaporation induced self-assembly under HCl conditions
Abstract
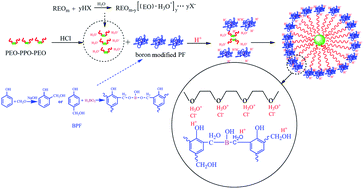
By using hydrochloric acid (HCl) as an acidity regulator, boron-doped ordered mesoporous carbon (B-OMCs) were synthesized via solvent evaporation induced self-assembly (EISA) method. And the self-assembly mechanism of the B-OMCs under HCl conditions was investigated. Not only low-molecular weight boron-modified phenolic resin (BPF), but also triblock copolymer F127 can be protonated by HCl, which results in enhancement of the interaction between precursor and template. Both double-layer hydrogen bonding and electrostatic Coulomb forces act as the driving force for self-assembly of B-OMCs under HCl conditions. In addition, the effect of HCl content on the mesostructure and character of the B-OMCs was studied. With the increase of HCl content, the pore size of B-OMCs decreases, while the surface area, pore order and boron content of B-OMCs increase initially, and then drop off gradually. When pH = 4, the obtained B-OMC has a well-ordered mesoporous structure, highest surface area (690 m2 g−1) and boron content (1.96 wt%). Besides, it possesses excellent electrochemical and capacitance performance (200 F g−1).


 Please wait while we load your content...
Please wait while we load your content...