Ring opening polymerization of lactides and lactones by multimetallic alkyl zinc complexes derived from the acids Ph2C(X)CO2H (X = OH, NH2)†
Abstract
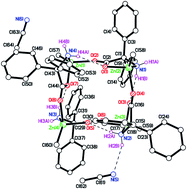
The reaction of the dialkylzinc reagents R2Zn with the acids 2,2-Ph2C(X)(CO2H), where X = NH2, OH, i.e. 2,2′-diphenylglycine (dpgH) or benzilic acid (benzH2), in toluene at reflux temperature afforded the tetra-nuclear ring complexes [RZn(dpg)]4, where R = Me (1), Et (2), 2-CF3C6H4 (3), and 2,4,6-F3C6H2 (4); complex 2 has been previously reported. The crystal structures of 1·(2MeCN), 3 and 4·(4(C7H8)·1.59(H2O)) are reported, along with that of the intermediate compound (2-CF3C6H4)3B·MeCN and the known compound [ZnCl2(NCMe)2]. Complexes 1–4, together with the known complex [(ZnEt)3(ZnL)3(benz)3] (5; L = MeCN), have been screened, in the absence of benzyl alcohol, for their potential to act as catalysts for the ring opening polymerization (ROP) of ε-caprolactone (ε-CL), δ-valerolactone (δ-VL) and rac-lactide (rac-LA); the co-polymerization of ε-CL with rac-LA was also studied. Complexes 3 and 4 bearing fluorinated aryls at zinc were found to afford the highest activities.


 Please wait while we load your content...
Please wait while we load your content...