Efficient esterification of n-butanol with acetic acid catalyzed by the Brönsted acidic ionic liquids: influence of acidity†
Abstract
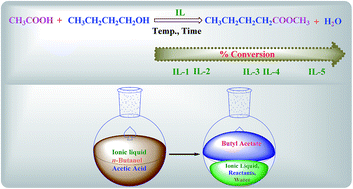
The efficiency in the catalytic performance of halogen free functionalized room-temperature Brönsted acidic ionic liquids (BAILs), having different acidities (due to the ILs containing zero, a single, and or a double –SO3H functional group), for the esterification of n-butanol with acetic acid under various reaction conditions was investigated. The synthesized BAILs have much weaker corrosiveness than that of H2SO4. The nature of both counter anion and cation as well as the presence of additional functional (–SO3H) groups influenced the behavior of the catalyst. Interestingly, the acidic character of the ILs facilitates the reaction under extremely mild conditions, with a short reaction time, and reduction in the side reactions; moreover, the liquid–liquid biphasic reaction mode leads to good yields. The physicochemical properties of these BAILs were characterized by a variety of different analytical and spectroscopic techniques, such as NMR, FT-IR, mass spectrometry, TGA, and UV-vis spectroscopy for the determination of Hammett acidity. In particular, IL-5 having the highest acidity demonstrated excellent catalytic activity for esterification. An additional advantage of BAILs is the simple procedure for the separation of product and catalyst, where the catalysts can be easily recycled without the loss of catalytic activities, making IL-5 an important alternative catalyst for a commercially viable esterification process.
- This article is part of the themed collections: Organic chemist’s toolbox, 2017-2018 Top Cited Research from China and Editors Collection for RSC Advances - India


 Please wait while we load your content...
Please wait while we load your content...