Synthesis and characterization of metallo-supramolecular polymers from thiophene-based unimers bearing pybox ligands†
Abstract
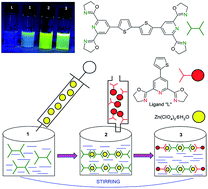
A series of novel metallo-supramolecular polymers was successfully prepared, based on 2,6-bis(2-oxazolinyl)pyridine building blocks consisting of pyridine flanked by two oxazoline rings as a tridentate binding site bridged with thiophene, bithiophene and thienothiophene as a linker, beginning from a cheap and commercially available 1,4-dihydro-4-oxo-2,6-pyridinedicarboxylic (chelidamic) acid. Metallo-supramolecular polymers were obtained and spectroscopically characterized upon treatment of the synthesized building blocks, also known as unimers, with the following metal ions: Fe2+, Zn2+, Ni2+, Cu2+. During the self-assembly process of the prepared unimers with a Cu2+ ion coupler, UV/vis investigation showed the highest shift of absorption maxima to lower energies, contrary to the Fe2+ ion couplers where the lowest value of shift was detected, compared to the free unimer. Upon the complexation of the Fe2+ ion coupler with selected unimers, the appearance was observed of new absorption bands around 600 nm ascribable to metal-to-ligand charge-transfer transitions. The luminescence study of the complexation of the synthesized unimers with Zn2+ exhibited a high fluorescence increase with an increase of metal ion concentration. Adversely, all of the other metals only showed fluorescence quenching.
- This article is part of the themed collections: Editors' collection: Supramolecular Polymers and Editors’ collection: Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...