OH- and O3-initiated atmospheric degradation of camphene: temperature dependent rate coefficients, product yields and mechanisms†
Abstract
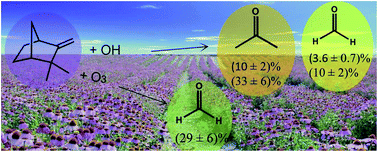
Gas-phase rate coefficients for the reactions of OH and O3 with camphene have been measured over the temperature range 288–311 K using the relative rate method. The experiments were carried out in an environmental chamber using long-path FTIR spectroscopy to monitor the reactants. Room temperature rate coefficients (in cm3 per molecule per s) of k(camphene+OH) = (5.1 ± 1.1) × 10−11 and k(camphene+O3) = (5.1 ± 1.1) × 10−19 were obtained for the OH and O3 reactions, respectively. The temperature dependence of the reactions are best fit by the Arrhenius expressions (in cm3 per molecule per s) k(camphene+OH) = (4.1 ± 1.2) × 10−12 exp[(754 ± 44)/T] for the OH reaction and k(O3+camphene) = (7.6 ± 1.2) × 10−18 exp[−(805 ± 51)/T] for the O3 reaction. To the best of our knowledge, this is the first report of the temperature dependencies for the reactions of OH and O3 with camphene. In addition, product studies have been performed at (298 ± 2) K and 760 Torr of synthetic air for the reaction of OH + camphene in the absence and presence of NOx, and for O3 molecules + camphene at (298 ± 2) K and 750 Torr of synthetic air. For the OH reaction the following molar product yields were obtained: acetone (10 ± 2)% and (33 ± 6)%, and formaldehyde (3.6 ± 0.7)% and (10 ± 2)% in the absence and presence of NOx, respectively. Formaldehyde with a molar yield of (29 ± 6)% was the only product uniquely identified and quantified for the O3 reaction.


 Please wait while we load your content...
Please wait while we load your content...