l-Cysteine electrooxidation in alkaline and acidic media: a combined spectroelectrochemical and computational study†
Abstract
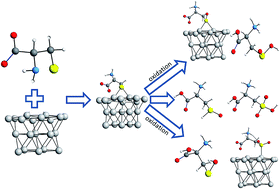
Due to the numerous possible oxidation states of sulfur, L-cysteine and L-cystine are very important amino acids that are utilized to understand the adsorption and redox activity of proteins and peptides. Therefore, the study of the adsorption/oxidation of these molecules can be used as a model system to understand protein behaviour. The aim of the present communication is to study this adsorption/oxidation process by infrared spectroscopy and DFT/PBE-D3/def2-SVP/COSMO (water) calculations. A bridge geometry (S bonded to two Pt atoms) was found to be the chemical adsorption link formed, and experimental data corroborate the DFT predictions. For both species, the surface–carboxyl group interaction is stronger at lower pH values, and the conformations of the adsorbed species were found to be dependent on the pH, with the amino group getting closer to the surface along with increases in the electrolyte alkalinity. The oxidation study was also performed at different pH values, and a Kolbe decarboxylation was found to be a side reaction at every pH, being more important in strong acidic solutions. Sulfur-containing adsorbed oxidized species were inferred based on the optimized structures of the adsorbed/desorbed species. A complex oxidation mechanism is proposed here, and species, such as sulfoxide, adsorbed sulfoxide, sulfenic and sulfinic acids and adsorbed sulfone, are suggested as intermediates in the oxidation of L-cysteine to sulfonic acid.


 Please wait while we load your content...
Please wait while we load your content...