Rhodium(i) diphenylphosphine complexes supported on porous organic polymers as efficient and recyclable catalysts for alkene hydrogenation†
Abstract
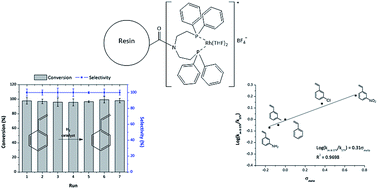
This paper describes the synthesis and characterization of porous polymeric materials as a support for rhodium(I) cationic coordination compounds and their use as heterogeneous catalysts for alkene hydrogenation. The synthetic strategy was the insertion of a vinyl-moiety in a bis(2-chloroethyl)amine precursor to provide highly porous resins with an enriched modifiable surface. The precursors synthesized were N,N-bis(2-chloroethyl)prop-2-en-1-amine (Alk-POL) and N,N-bis(2-chloroethyl)acrylamide (Acy-POL). The resins were obtained through suspension polymerization of methyl acrylate and divinylbenzene as a co-polymer and cross-linker, respectively. The resin surfaces were functionalized with diphenylphosphine groups followed by Rh(I) metal deposition using [Rh(COD)2]BF4 (COD = 1,5-cyclooctadiene) as the catalyst precursor. The Rh-catalysts were characterized by different physicochemical techniques and assessed for their catalytic performances in the heterogeneous hydrogenation of styrene and its derivatives. It was found that the catalytic activities and selectivity of the heterogenized rhodium complex (Rh-Alk-POL and Rh-Acy-POL) in the hydrogenation reactions were comparable to its homogeneous analogue. Analysis of the spent homogeneous resin Rh-Alk-POL catalyst after the first reaction cycle showed the presence of metallic Rh nanoparticles arising from the reduction of the Rh complex. Extensive recycling and Rh leaching studies were carried out for the Rh-Acy-POL catalyst. Both the activity and selectivity could be maintained for at least seven reaction runs and without metal leaching during the reaction cycles. We have also studied the liquid-phase hydrogenation reaction of various styrene m-substituted derivatives. The Rh-Acy-POL catalyst exhibits excellent catalytic activity for hydrogenation of the substrates and only vinyl-group hydrogenation was detected. Finally, the presence of electron-donating/-withdrawing substituents at the meta-position resulted in different rates of vinyl group hydrogenation. This effect was quantified in terms of the Hammett relationship, in which the catalyst displayed a linear correlation between the Hammett substituent constant (σmeta) and the hydrogenation rate.


 Please wait while we load your content...
Please wait while we load your content...