Design, synthesis and antitubercular evaluation of benzothiazinones containing an oximido or amino nitrogen heterocycle moiety†
Abstract
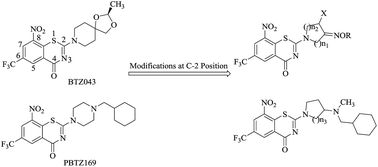
A series of 8-nitro-6-(trifluoromethyl)-1,3-benzothiazin-4-ones (BTZs) bearing an oximido or amino nitrogen heterocycle moiety through modifications at the C-2 position of BTZ043 and BPTZ169 were designed and synthesized as new antitubercular agents. Many of the target compounds demonstrate excellent in vitro activity (MIC: <0.016–0.088 μg mL−1) against the drug susceptive H37Rv strain and two clinically isolated multidrug-resistant Mycobacterium tuberculosis (MTB) strains. Compound 10a displays acceptable safety, aqueous solubility and pharmacokinetic properties, opening up a new possibility for further development.


 Please wait while we load your content...
Please wait while we load your content...