Phosphoric acid doped hydrophobic ionic liquid-based composite membranes for anhydrous proton exchange membrane application
Abstract
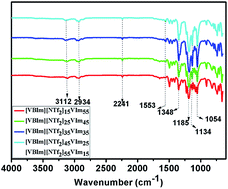
In this study, phosphoric acid doped hydrophobic ionic liquid-based composite membranes are successfully synthesized and characterized. 1-Vinyl-3-butylimidazolium bis(trifluoromethylsulfonyl)-imide ([VBIm][NTf2]) was synthesized and used as hydrophobic phase in the composite membranes. The H3PO4 uptake of the composite membranes increases with the increasing content of [VBIm][NTf2] and then decreases. The resultant composite membranes showed good thermal stability, mechanical properties and high proton conductivity (up to the order of 10−2 S cm−1 at 180 °C) at high temperatures under anhydrous conditions. The results of this study suggest that this type of PEMs have good perspectives for high temperature proton exchange membrane fuel cell applications.

 Please wait while we load your content...
Please wait while we load your content...