A series of coordination polymers based on terphenyl tetracarboxylates and bis-pyridyl ligands with water vapor sorption properties†
Abstract
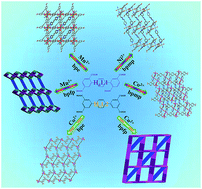
Six new coordination polymers, namely, {[Mn2(L1)(bpfp)(H2O)2]·6H2O·(CH3OH)}n (1), {[Mn4(μ3-O)2(L1)2(bpe)2(H2O)2]·2H2O}n (2), [Ni(HL1)(Hbpmp)(H2O)2]n (3), [Co2(μ2-OH)(HL1)(bpmp)(H2O)4]n (4), {[Co2(L2)(bpfp)(H2O)2]·4H2O}n (5), {[Co2(L2)2(bpe)2(H2O)2·2H2O]·(NH4)4}n (6) (H4L1 = [1,1′:4′,1′′-terphenyl]-2,2′′,5,5′′-tetracarboxylic acid, H4L2 = [1,1′:4′,1′′-terphenyl]-3,3′′,5,5′′-tetracarboxylic acid, bpfp = bis(4-pyridylformyl)piperazine, bpe = 1,2-bis(pyridin-4-yl)ethane and bpmp = N,N′-bis(4-pyridyl)piperazine) were synthesized under hydrothermal conditions by terphenyl tetracarboxylates, bis-pyridyl ligands and transition metal salts. These six complexes were characterized by elemental analysis, infrared (IR) spectroscopy, thermogravimetric analysis (TGA), single-crystal X-ray diffraction, powder X-ray diffraction (PXRD) and fluorescence spectroscopy. Complexes 1 and 5 possess three-dimensional (3D) (4,6)-connected networks with {43·63}2{46·66·83} and {44·610·8}{44·62} topologies, respectively. Complexes 2–4 and 6 feature two-dimensional (2D) networks, which are expanded into 3D supramolecular frameworks via hydrogen bonding interactions. Desorption of lattice water molecules in complexes 1 and 2 was analyzed, and the values of water vapor uptake are 15.17 mL g−1 and 27.24 mL g−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...