Continuous flow-ultrasonic synergy in click reactions for the synthesis of novel 1,2,3-triazolyl appended 4,5-unsaturated l-ascorbic acid derivatives†
Abstract
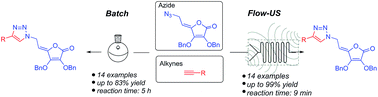
A combination of flow chemistry and batch-based synthetic procedures has been successfully applied to the assembly of novel 4,5-unsaturated L-ascorbic acid series 6a–6n with diverse C-6-substituted 1,2,3-triazole moiety. We report herein the first Cu(I)-catalyzed 1,3-dipolar cycloaddition of azido functionalized L-ascorbic acid derivative and selected alkynes to provide target 1,2,3-triazolyl appended 4,5-didehydro-5,6-dideoxy-L-ascorbic acid library 6a–6n under both micro-flow and batch conditions. Implementation of ultrasound with flow chemistry accelerated hour-scale reaction conditions in batch to the minute range in micro-flow device and considerably improved the yields for the flow syntheses of 6a–6n. Moreover, the synergistic use of microreactor technology and ultrasonic irradiation highlights the sustainable eco-friendly aspect of utilized method.
- This article is part of the themed collection: A Decade of Progress in Click Reactions Based on CuAAC

 Please wait while we load your content...
Please wait while we load your content...