Study on the curing reaction kinetics of a novel epoxy system
Abstract
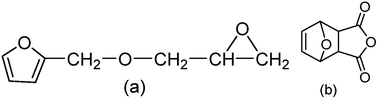
The anhydride curing agent of 3,6-enodro-1,2,3,6-tetrahydrophthalic anhydride (OBPA) and the reactive epoxy diluent of furfuryl glycidyl ether (FGE) were prepared and characterized by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (1H NMR). The curing reaction kinetics process of an EP/OBPA/FGE epoxy system was studied by non-isothermal DSC methods. The parameters of the kinetics were calculated using the Kissinger model, Crnae model, Ozawa model and β–T (temperature–heating speed) extrapolation, respectively. In addition, the effect of FGE on the thermomechanical properties (glass transition temperature) and mechanical properties (flexural strength and the tensile strength) in the EP/OBPA/FGE were studied, indicating that when the content of FGE was 10 wt% the epoxy system reaches the best mechanical properties.

 Please wait while we load your content...
Please wait while we load your content...