Synthesis of chiral R/S-pseudopeptide-based Cu(ii) & Zn(ii) complexes for use in targeted delivery for antitumor therapy: enantiomeric discrimination with CT-DNA and pBR322 DNA hydrolytic cleavage mechanism†
Abstract
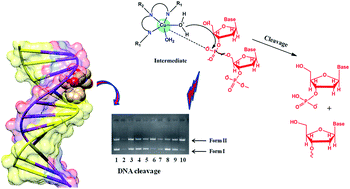
Chiral pseudopeptide Cu(II) and Zn(II) complexes (1S/R and 2S/R, respectively), were obtained by a de novo synthetic strategy employing pseudopeptide synthons derived from R/S-2-amino-2-phenylethanol and N-methyliminodiacetic acid. The complexes were thoroughly characterized by elemental analysis, mass spectrometry, and IR; the 2S/R complexes were further characterized by 1H, 13C NMR, whereas 1S/R complexes were studied by EPR spectroscopy. In vitro DNA binding studies were carried out by UV-Vis, fluorescence, thermal denaturation and circular dichroic techniques. The experimental results revealed that the complexes strongly bind to DNA via electrostatic interaction. The extent of binding was quantified by computing their intrinsic binding constant (Kb) and binding constant (K) values, which showed that the S-enantiomers of both complexes 1 and 2 exhibited higher binding propensities as compared to their R-enantiomeric analogs, and followed the trend 1S > 2S > 1R > 2R. Thermal denaturation studies of complexes in the absence and presence of CT-DNA have been carried out and the calculated ΔTm was found to be 1–3 °C, depicting the electrostatic mode of binding, which corroborated the results of the UV-Vis, fluorescence and other optical methods. The cleavage efficiencies of 1S and 2S with pBR322 DNA were evaluated by gel electrophoretic assay. S-Enantiomers of both Cu(II) and Zn(II) complexes were found to be efficient cleaving agents and cleavage reactions were mediated by hydrolytic pathways, which were further validated by relegation experiments using the T4 ligase enzyme. The cytotoxic activity of 1S and 2S showed pronounced GI50 values <10 μg mL−1 in the case of the HeLa cancer cell line, whereas for other cell lines, viz. MCF7, Hep-G2 and MIA-Pa-Ca-2, moderate activity was observed, which implicated the selective response of drug entities towards different cancer phenotypes.


 Please wait while we load your content...
Please wait while we load your content...