Small hybrid heteroaromatics: resourceful biological tools in cancer research
Abstract
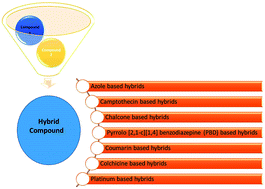
Nowadays, hybrid drugs containing two or more covalently linked known potential pharmacophores are designed to simultaneously modulate multiple targets of multifactorial diseases to overcome the side effects associated with a single drug. In this review, an overview of the design strategies employed by various scientists over the past 20 years has been presented. The overview includes the synthesis of different chemical structure-based anticancer hybrids using molecular hybridization techniques. To tackle one of the world's most devastating diseases such as cancer, researchers have exploited the molecular hybridization (MH) technique to synthesize different anticancer hybrids, which include hybrids based on azole, camptothecin, chalcone, pyrrolobenzodiazepine (PBD), coumarin, colchicine, platinum, and some miscellaneous structures. The selection of two or more moieties for generating the hybrid drug is generally aided by the observed (or anticipated) synergistic or additive pharmacological activities of each single moiety. This eventually leads to the identification of novel and better active chemical entities with a superior profile as compared to the parent moieties. In addition to the design strategies, this review also highlights the structure–activity relationship (SAR), mechanism of action, and key features of the synthesized anticancer hybrids.
- This article is part of the themed collection: 2017 Review articles


 Please wait while we load your content...
Please wait while we load your content...