Protein–inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in l-xylulose production†
Abstract
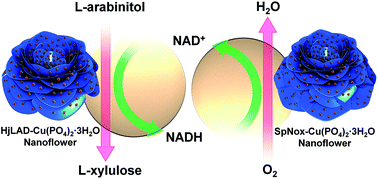
The facile synthesis of protein–inorganic hybrid nanoflowers was evaluated for the efficient immobilization of recombinant his-tagged enzymes, which have a broad range of potential applications. In this study, we report the preparation of a metal–protein hybrid nanoflower system for efficient immobilization of the recombinant enzymes L-arabinitol 4-dehydrogenase from Hypocrea jecorina (HjLAD) and NADH oxidase from Streptococcus pyogenes (SpNox). Compared with free enzymes, synthesized hybrid nanoflowers exhibited enhanced enzymatic activities of 246 and 144% for HjLAD and SpNox, respectively. We have demonstrated that immobilized enzymes retained high catalytic activity and improved the tolerance towards pH and temperature changes. Synthesized nanoflowers also retained high storage stability and reusability. In addition, the immobilized enzymes exhibited significantly enhanced L-xylulose production under co-factor regeneration conditions than the free enzyme combination. These results demonstrate that variations in the concentration of metals and synthesis conditions of nanoflowers can be extended to efficiently immobilize recombinant his-tagged enzymes.


 Please wait while we load your content...
Please wait while we load your content...