Pd-Catalyzed cycloisomerization/nucleophilic addition/reduction: an efficient method for the synthesis of spiro-pseudoindoxyls containing N,N′-ketal†
Abstract
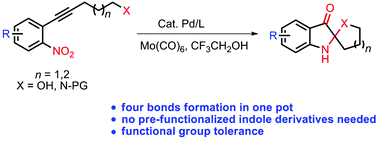
An efficient method for the synthesis of C2-spiropseudoindoxyls which are common structural units prevalent in indole alkaloids was developed. A study of the mechanism indicated that this protocol involved a Pd-catalyzed 5-exo-dig nitroalkyne cyclization and an internal N–O bond redox process. The fungicidal activity evaluation of representative compounds highlighted this reaction for the construction of bioactive functionalized spiro-heterocycles.


 Please wait while we load your content...
Please wait while we load your content...